PartitionBio technology enables
superior delivery
of biologics to cells
RNA, DNA, and proteins
Seamless cell delivery
as easy as A B C
-
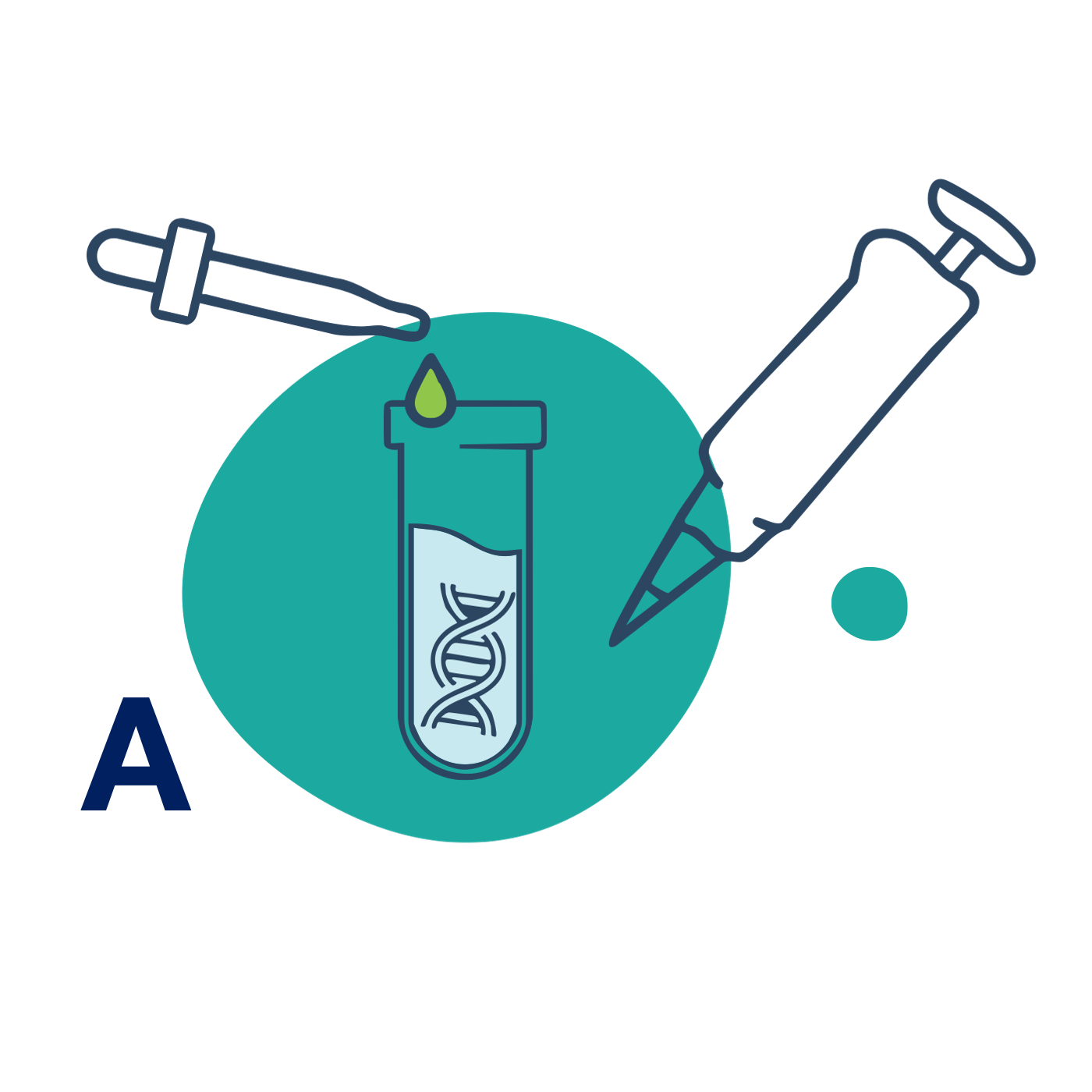
A – Assemble
Mix your cargo with BubbleFect in water – done.
Simply combine your nucleic acid or protein cargo with BubbleFect. Mix by pipetting or vortexing.
💡 First-time users can run a quick titration to optimise for their system. -
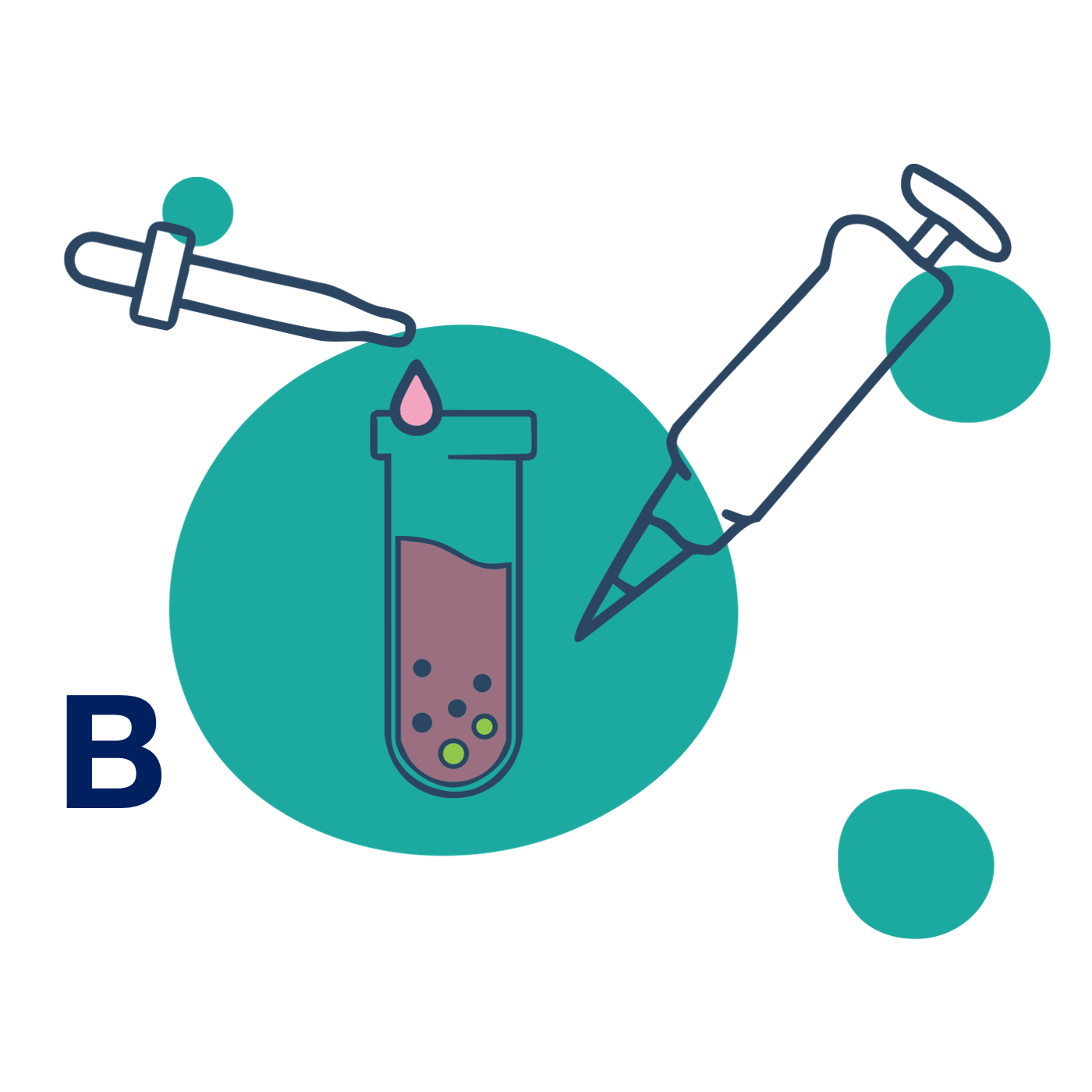
B – Bubbles
Add serum-free medium to instantly create Bubbles.
Pipette in serum-free medium – Bubbles form on contact. The solution turns cloudy and is ready to use, no incubation needed. -
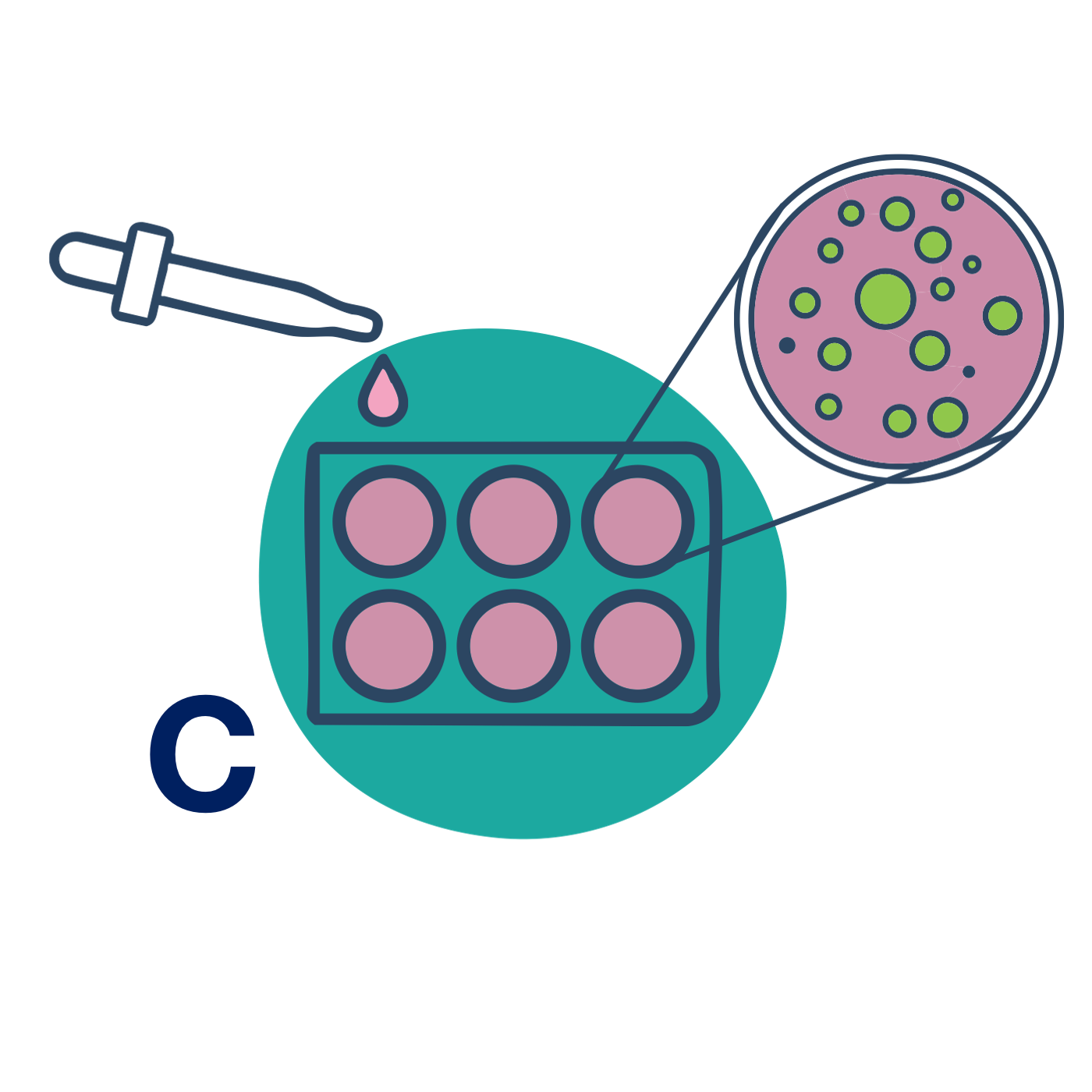
C – Cells
Transfer Bubbles onto your cells – that’s it.
Add an equal volume of Bubbles to cells. Incubate for 1 hour. Optional: wash cells in rich medium and proceed with your standard assay protocol.
Why it works
BubbleFect integrates into existing protocols with no disruption. It’s fast and requires little optimisation. Making high-efficiency delivery accessible for any laboratory.
FULL PROTOCOL

A new phase in cell delivery
From
£80
Breaking Barriers
Why Cell Delivery Is Holding Back the Future of Therapeutics
The cell delivery market faces significant scientific and technical challenges that hinder the effective translation of many advanced therapeutics. Viral vectors, while historically dominant, present risks such as insertional mutagenesis, immunogenicity, and limited cargo capacity, AAVs, for example, struggle with packaging larger gene-editing systems like CRISPR-Cas9. Non-viral methods, such as lipid nanoparticles (LNPs), often induce cytotoxicity and inflammatory responses due to ionisable lipid components, and are poorly suited to transfecting hard-to-target primary or immune cells. Electroporation, though widely used in cell therapy, can cause irreversible membrane damage and drastically reduce cell viability, especially in sensitive cell types like T cells or stem cells. Furthermore, many current delivery systems are constrained by temperature sensitivity, requiring cold chain logistics that increase cost and limit accessibility. Collectively, these issues present major bottlenecks in therapeutic development, particularly for applications requiring repeated dosing, systemic delivery, or regulatory scalability.
How PartitionBio is changing that
Despite major advances in cell and gene therapies, the delivery of biologics into cells remains a critical scientific bottleneck. At PartitionBio, we’re addressing this challenge with a fundamentally new approach. Our proprietary BubbleFect technology uses principles of liquid–liquid phase separation (LLPS) to deliver a broad range of biologics, LIKE mRNA, proteins, CRISPR components, into cells safely and efficiently. BubbleFect is non-viral, room-temperature stable, and compatible with sensitive cells, offering a delivery solution that is as scalable and accessible as the therapies it enables.
Smart, Gentle Delivery for Advanced Biologics
PartitionBio’s proprietary delivery platform engineered to overcome the limitations of traditional intracellular delivery. Built on the principles of liquid–liquid phase separation (LLPS), BubbleFect uses custom-designed biocompatible biopolymers that self-assemble with biologic cargo, such as mRNA, proteins, or CRISPR components, into aqueous microdroplets.
In simple mixing steps, the biologic cargo and BubbleFect reagents spontaneously self-sort into enriched, membrane-less droplets through phase separation. These droplets act as dynamic, cell-compatible compartments that concentrate the cargo without chemical modification or harsh conditions. The result is a highly efficient, room-temperature-stable delivery system that mimics natural cellular compartmentalisation.
Once applied to cells, these droplets are readily absorbed via endocytic or fusion-like mechanisms, enabling the direct and gentle release of biologic payloads into the intracellular environment. This mechanism avoids the membrane damage and toxicity associated with electroporation or lipid-based carriers and allows delivery into a wide variety of hard-to-transfect cell types, including primary immune cells and stem cells, making BubbleFect a powerful tool for both research and therapeutic applications.

A new phase in cell delivery
From
£80
-

Optimised for High-Throughput Screening
High Throughput Screening has revolutionised drug discovery and biochemical research by enabling rapid evaluation of vast compound libraries and functional genomics screens to identify promising candidates for further development. Despite its power, HTS still faces challenges, such as drug solubility, effective cell delivery, and high costs. BubbleFect is simple to use, room temperature stable cell delivery vehicles for a wide variety of cargos, including large macromolecules (DNA, RNA, protein, CRISPR, etc) and furthermore can act as a suitable solvent environment for otherwise insoluble small molecule drugs.
-

Powerful in Functional Genomics Workflows
Functional genomics seeks to decode gene functions and interactions by manipulating gene expression across a range of cell types. BubbleFect advances this field by offering superior transfection efficiency, low cytotoxicity, compatibility with various nucleic acids, and stability at room temperature. These features enable researchers to carry out more reliable, scalable, and high-throughput gene modulation studies, accelerating discoveries in disease mechanisms and therapeutic development.
-

CRISPR-Ready with Low Toxicity & High Consistency
BubbleFect uses molecular condensates; membrane-less compartments formed through phase separation. BubbleFect is emerging as powerful enhancer for CRISPR genome editing. By concentrating CRISPR components, improving specificity, and enabling programmable control, BubbleFect unlocks new applications in gene therapy, functional genomics, and synthetic biology. This innovative fusion of technologies offers a pathway to more efficient, targeted, and responsive genome engineering.
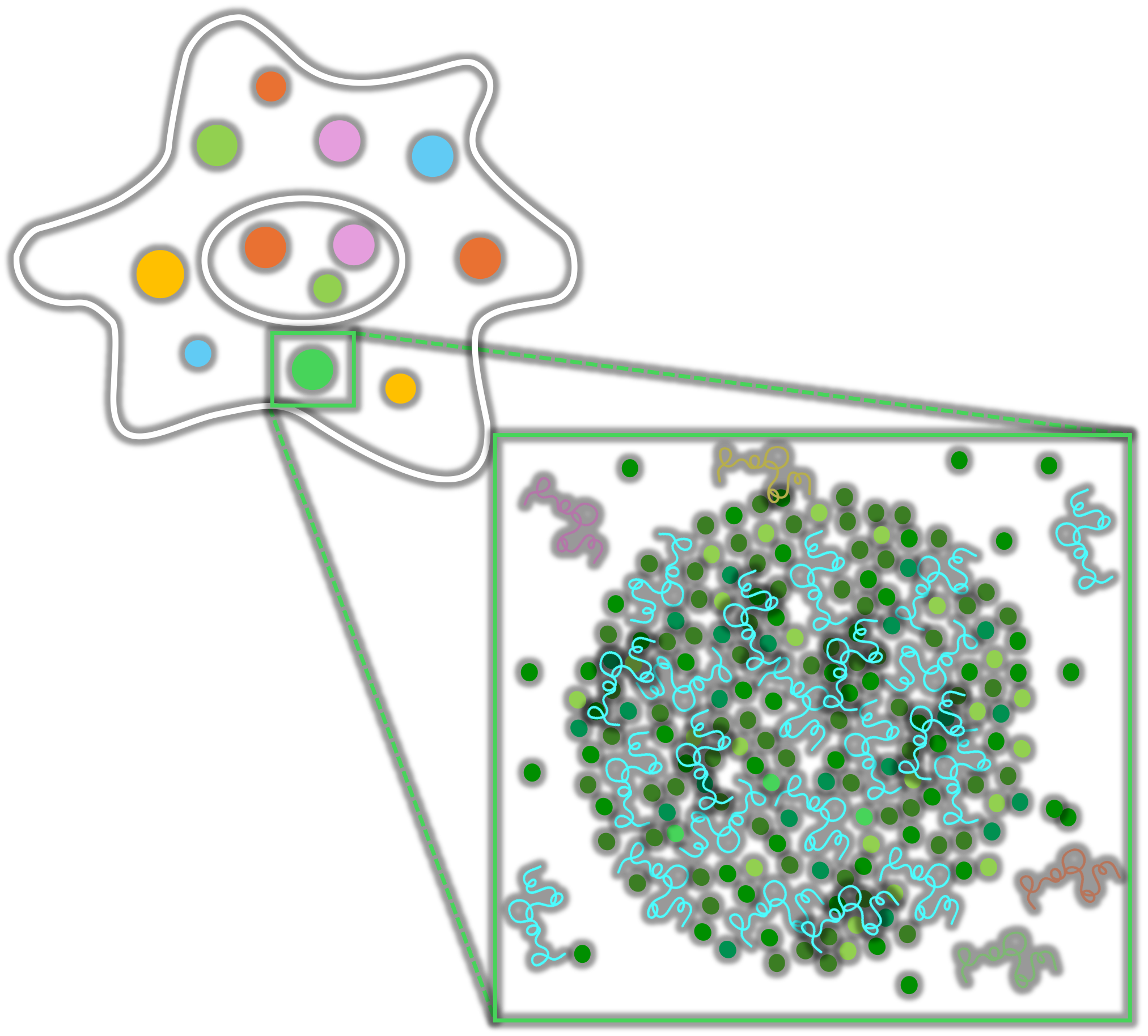
What Is Liquid–Liquid Phase Separation?
Liquid–liquid phase separation (LLPS) is a natural process where proteins and nucleic acids organise into membrane-less droplets inside cells, like oil separating from water. These dynamic structures, called biomolecular condensates, form and dissolve quickly, helping cells control vital processes like gene expression, stress response, and signal regulation.
At the molecular level, LLPS happens when specific molecules stick together through multivalent interactions, concentrating biological activity without needing a surrounding membrane. This self-assembly principle enables highly efficient, reversible compartmentalisation, a breakthrough concept now being harnessed to build smarter tools for drug delivery and cell engineering.
Highly Efficient, Targeted Compartmentalisation
LLPS allows cells to rapidly organise molecules into dynamic, membrane-less compartments. This enables precise control of biochemical reactions, concentrating key proteins, RNAs, or enzymes exactly where they’re needed, when they’re needed.
Reversible and Tunable by Design
Unlike membrane-bound organelles, LLPS-based condensates can form and dissolve on demand in response to changes in temperature, pH, or concentration. This makes them ideal for designing delivery systems that are responsive, non-permanent, and compatible with live-cell applications.
Applications: Unlocking Therapeutic Potential
Nucleic Acid Delivery
PartitionBio applies its expertise in biomolecular condensates to unlock safer, more efficient delivery of nucleic acid based cargos, such as mRNA, DNA, siRNA, ASO and more. By leveraging LLPS principles, we enable dynamic, compartmentalised delivery systems, like BubbleFect, that enhance mRNA stability, translation efficiency, and intracellular targeting. This approach reduces reliance on complex cold chains and toxic carriers, helping mRNA therapies scale from lab to clinic with greater flexibility and reach.
Gene Editing and CRISPR Modulation
Using LLPS-driven delivery systems, PartitionBio supports precise, non-viral intracellular delivery of CRISPR-Cas components. Thanks to the ability to mix-and-match cargo types, BubbleFect users will have the opportunity to explore the delivery of gene editing complexes as an RNP, which is an approach that is reported to reduce off-target editing.
Protein and Enzyme Replacement Therapies
PartitionBio’s phase separation technology provides a unique route for delivering fully folded, functional proteins directly into cells. This enables real-time modulation of intracellular pathways, enzyme replacement in genetic disorders, intracellular antibody delivery, and novel therapeutic strategies that bypass transcription altogether. The result is efficient, gentle intracellular access that maintains protein integrity and cell viability.
Advanced Research Tools and Cell Engineering
By rethinking how molecules are packaged and delivered, using LLPS principles, PartitionBio empowers researchers to engineer cells more effectively. Whether for functional genomics, high-throughput screening, or therapeutic cell line development, our platforms simplify the delivery of nucleic acids and proteins across a wide range of cell types, enabling faster results without the need for specialised equipment or protocols.

A new phase in cell delivery
From
£80
Supporting Data: See the Science
eGFP mRNA expression in non-dividing terminally differentiated muscle myotube cells
In comparative studies, BubbleFect demonstrated a significant improvement in transfection efficiency, increasing the reporter protein level to ten times higher than that achieved by a leading lipid-based competitor under identical conditions. This increase in yield reflects the higher payload delivery that is possible with BubbleFect, with minimal toxicity or disruption to cell health. The result is superior performance, especially in applications requiring high expression levels, broader cell coverage, or sensitive cell types. BubbleFect’s efficient, reagent-only workflow also makes it easier to reproduce these results across labs and experiments.
Delivering Antibodies Inside Cells to Unlock New Frontiers in Research and Therapeutics
BubbleFect enables efficient intracellular delivery of targeting antibodies, expanding the potential of antibody-based research and therapeutics beyond surface markers and secreted proteins. This opens the door to entirely new applications, including functional proteomics and subcellular targeting, powered by data from resources like the Human Protein Atlas. With BubbleFect, intracellular delivery of charge-neutral cargos such as proteins of any class is no longer a barrier — it's a breakthrough.